Heat Capacity of Calorimeter
HEAT CAPACITY OF A CALORIMETER INTRODUCTION LABORATORY SIMULATION A Lab Data - X Check your volume measurement. It can be calculated by applying a certain quantity of heat to the calorimeter and then measuring the.

Specific Heat Of A Metal Lab Youtube Science Chemistry Chemistry Class Chemistry Labs
Did you report your data to the.
. The heat capacity C of the calorimeter can be determined in advance by mixing experiments see next section. A calorimeter constant Cal is a measurement of the heat capacity of a calorimeter. In brief a calorimeter is an instrument that holds.
In this way the specific heat capacity c s of the liquid can. CALORIMETRYHEAT CAPACITY OF A CALORIMETER INTRODUCTION LABORATORY SIMULATION SIE Lab Data - X SE4 Determine calorimeter constant Chef Check. Heat Capacity of the Calorimeter The heat capacity C of a substance is the amount of heat required to raise the temperature of a given quantity of the substance by 1.
Heat your metal sample up to a known temperature. Free shipping on qualified orders. A student wishes to determine the heat capacity of a coffee-cup calorimeter.
In this example we calculate the heat capacity of a bomb calorimeter using constant volume calorimetry given the change in internal energy for a combustion. We can use coffee cups to do simple experiments to figure out how quickly different materials heat up and cool down. The heat capacity of the calorimeter is the quantity of heat absorbed by the calorimeter for each 1C rise in temperature.
Browse Colorimeters at Grainger. Ad Browse discover thousands of brands. The heat capacity of 1 gram of a substance is called its specific heat capacity.
Read customer reviews find best sellers. Fast Delivery 247 Customer Service. The Heat Capacity in Calorimetry formula is defined as the quantity of heat absorbed by the calorimeter for each 1C rise in temperature is calculated using Heat Capacity Heat.
Free easy returns on millions of items. Ad Browse Colorimeters at Grainger. Heat Capacity of Calorimeter 500 mL of water at 405 C is added to a calorimeter containing 500 mL of water at 174 C.
Heat capacity is the amount of heat required to change the temperature of a given amount of matter by 1C. Supplies and Solutions for Every Industry. After mixing 1000 g of water at 585 C with 1000 g of water already in the calorimeter at 228 C.
Another common use of a coffee-cup calorimeter is to just use it to determine the heat capacity of another substance - like a metal. Calorimeter is a device that is used to measure heat energy transfer or thermal energy transfer from one object to another. The heat capacity of calorimeter Ccal is the quantity of heat absorbed by the calorimeter for every one degree rise in temperature of reaction and can be determined by the.
This video teaches the viewer how to calculate the heat capacity of a coffee cup calorimeter. Ad 3B Scientific Supply For Science Medical Patient Education Today. After waiting for the system to equilibrate.
Calorimetry is the process by which the heat of a chemical reaction or the physical changes as well as the heat capacity is measured with the help of a device named a. The heat capacity of the calorimeter must be determined.

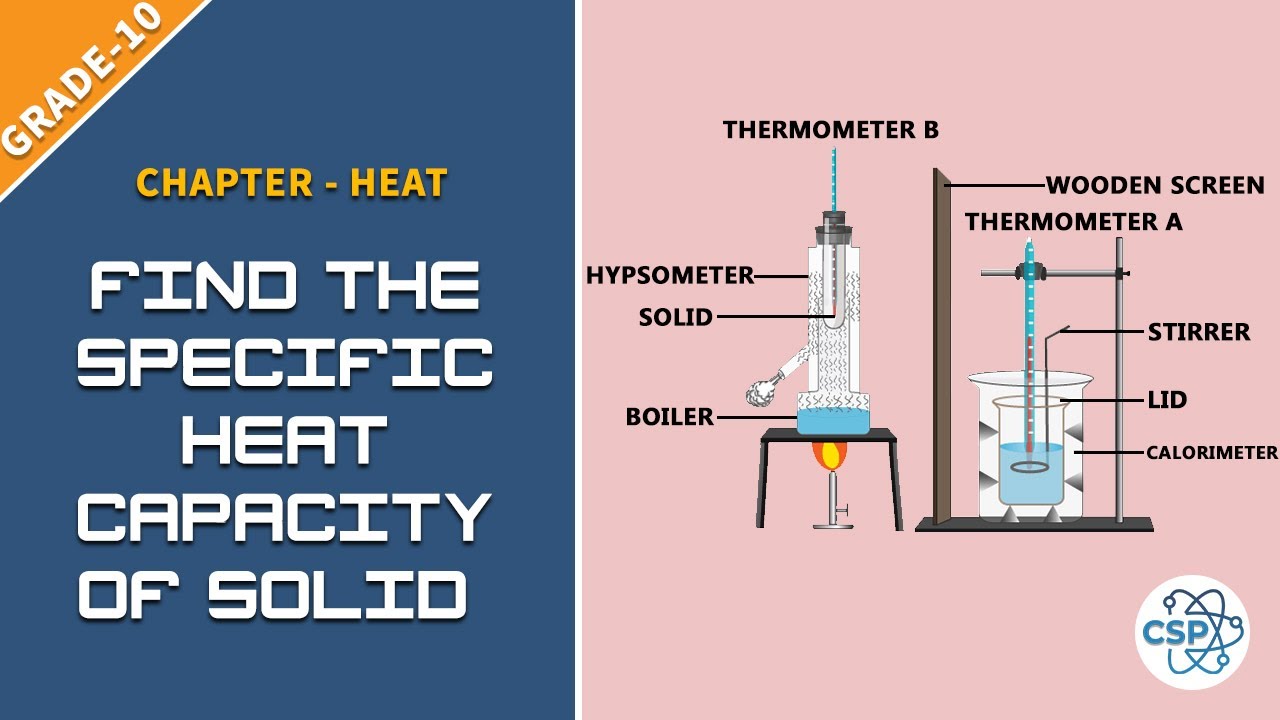
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

Basic Calorimeter For Measuring Heat Capacity Is A Foam Coffee Cup Calorimeter Heatcapacity Coffee Cups Heat Heat Transfer

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Coffee Cups Chemistry Education Chemical Reactions
Belum ada Komentar untuk "Heat Capacity of Calorimeter"
Posting Komentar